How does Soap Clean
- Diane Viall
- May 10, 2024
- 2 min read

A bit of scientific fun!
So, how does soap clean, we all know what a bar of soap looks like and why we use use it but do you know how it works.
To make soap you need two basic things - oils/butters and lye. You can use plant oils or animals oils depending on the type of soap you wish to make.
The Lye is a blend of Sodium Hydroxide and liquid, usually water.
When the oils/butters are blended together Saponification takes place - so it's simply the process of making soap. Easy hey? So what is Saponification then.
What is Saponification
I'll try to explain as easily as I can.
It's a reaction where upon an ester* is hydrolyzed into an alcohol and a carboxylic acid salt with the addition of an aqueous base.
*esters - are derived from carboxylic acids and alcohols and can be made into fats and oils by losing water.
So, what is saponification in laymans terms - well it's the chemical breakdown of oils/butters due to the reaction of water when Sodium Hydroxide (NaOH)is used to produce soap and alcohol.
They are just sodium salts of long-chain fatty acids or triglycerides that produce glycerol and fatty acid salt called SOAP.
Triglycerides are usually plant oils or animal fats and when they react with Sodium Hydroxide a hard bar of soap is created. Glycerine is produced as a by product during the process.
Why does Soap Cleanse - How does Soap Clean
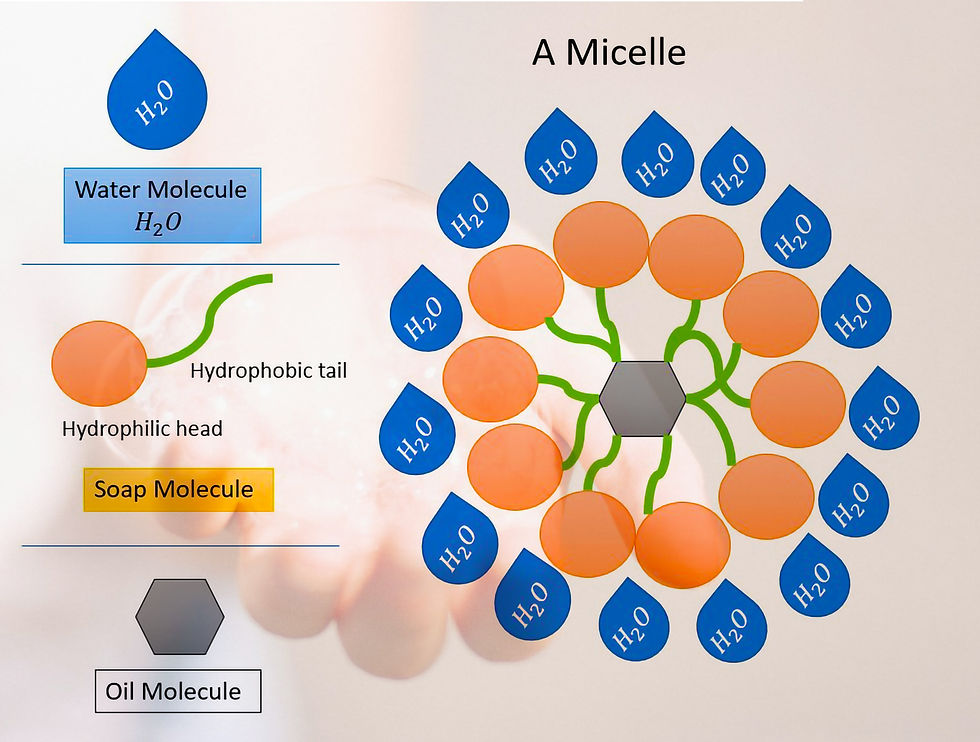
A soap is a molecule which has two different ends :
Hydrophylic - Water Attracting
Hydrophobic - Water Repellant
The soap molecules form structures called Micelles.
Most of dirt is oily in nature which does not dissolve in water, so when you wash your hands with a soap bar the hydrophobic tail, repels the water to reach the dirt while the hydrophilic end points towards the water and the dirt is trapped within the soap micelle. See above diagram of the micelle showing how soap cleanses.
Oils/Butters we have already established are fatty acids called triglycerides and we can choose different oils/butters to match our skin or help skin problems i.e. dryness, flaky skin, oily, mature skin, teenage skin etc. They are called :
Lauric
Myristic
Palmitic
Stearic
Ricinoleic
Oleic
Linoleic
Linolenic
These triglycerides will give your soap qualities like hardness, cleansing, skin conditioning, type of bubble (large, small and the amount) and creaminess.
I'm not a scientist but I hope I have been able to explain what happens when we make soap and why we get clean when we use it. Not only can soap clean our skin, it is very good at removing stains from our clothes as well. Spray the stain with water and just rub your bar over the stain gently before putting in the washing machine.
Happy soaping and happy cleansing.
x Diane


Easily understood